I riassunti , gli appunti i testi contenuti nel nostro sito sono messi a disposizione gratuitamente con finalità illustrative didattiche, scientifiche, a carattere sociale, civile e culturale a tutti i possibili interessati secondo il concetto del fair use e con l' obiettivo del rispetto della direttiva europea 2001/29/CE e dell' art. 70 della legge 633/1941 sul diritto d'autore
Le informazioni di medicina e salute contenute nel sito sono di natura generale ed a scopo puramente divulgativo e per questo motivo non possono sostituire in alcun caso il consiglio di un medico (ovvero un soggetto abilitato legalmente alla professione).
Notes: Chemistry, Matter, Scientific Method, Units, Energy, Temp, Density, Phases
Reading for this set of notes: All of Chapter 1, Appendix I and II
Suggested extra problems for text: 1 - 79
Chemistry:
What is Chemistry? The study of properties of matter and electron actions.
Chemistry is called the central science. This is because it links all other areas of science.
All actions in the universe are controlled by physics: on a large scale - an auto accident; on a small scale - the formation of a salt when water evaporates from the ocean.
Technically, all biological processes are run by physics, the processes are simply following laws of physics. Physics and biology are at far ends of the science spectrum. Chemistry bridges that gap. You can use chemistry to link any of the other sciences. Geology to physics, pharmacy to biology, oceanography to anthropology, mechanical engineering to physiology.
Electrons are the key to chemistry; they are the portion of the atom doing all the reacting. Chemistry deals with the exterior of the atom, the electrons, not the nucleus. The nucleus is the interior of atoms. The nucleus, as far as chemistry goes, is unaffected by chemical reactions.
Matter:
The definition of matter is, anything that has mass and occupies space. So, there are two parts to matter. It must occupy space and it must have mass. Volume is the unit used to measure the amount of space an object occupies. You can think of the volume unit in terms of how big something is, like a hot air balloon occupies a lot of space.
The other half of the definition deals with the term mass. Mass is a measure of the amount of matter. This differs from the size of the matter, the volume. The unit for mass is grams. Mass differs from weight, even though we, incorrectly, use them interchangeably.
Matter has mass. If it is near a large celestial body, i.e. Earth or the moon, it will have weight. Matter always has mass, it does not always have weight, i.e. you are in space. A chunk of matter’s weight will change based on its location, but its mass will not.
For example, a Frisbee with a mass of 175 grams will have 175 grams of mass on the moon and on Earth. But, on Earth it will weigh about 6 ounces, on the moon it will weigh about 1 ounce.
Pure substances and mixtures:
Matter is divided up into two broad categories.
Pure substances:
have fixed properties.
are composed of a constant composition.
cannot be broken apart physically.
Mixtures:
are properties vary with composition.
have proportions of the components vary throughout mixture.
can be physically separated into two or more pure substances.
Elements and Compounds:
Pure substances are divided up into two categories.
Elements:
are composed of one type of atom.
cannot be broken apart chemically.
Compounds:
are composed of more than one type of atom chemically bonded, called a molecule.
can be broken apart chemically, sometimes this is VERY difficult to do, sometimes not.
have pieces that are either individual atoms or smaller molecules.
cannot be broken by physical means.
you cannot hit the molecule and break the bonds or pull them apart or filter them apart.
Heterogeneous and homogeneous mixtures:
Mixtures can be separated by physical means like filtration or evaporation.
Mixtures are divided into two categories.
Heterogeneous properties are not the same throughout the mixture
Homogeneous properties are the same throughout the mixture
Elements:
Element: A pure substance composed of only one type of atom
Elemental Symbol: A 1 or 2 letter symbol assigned to each type of atom.
Only 11 have non-English origins.
IUPAC – International Union of Pure and Applied Chemistry, the governing body for chemical nomenclature. When new elements are made they go by Greek numbers, example, Uun, un un nil, 110, until a name is accepted.
Molecular Formula:
The molecular formula identifies which atoms and how many of these atoms will be found in each molecule. The atomic symbols identify the atoms and a subscript identifies the number of that atom. H2O is the molecular formula for water. Water is composed of hydrogen and oxygen. Each water molecule is composed of 2 hydrogen atoms and 1 oxygen atom. Since there is only 1 oxygen atom, a 1 will not be written; it is assumed that there is at least one oxygen, otherwise the O would not have been included in the formula.
Sulfuric acid has a molecular formula of H2SO4. Each molecule is composed of 2 hydrogen atoms, 1 sulfur atom and 4 oxygen atoms. Again, since there is only 1 sulfur, the number 1 is not needed as it is assumed.
Chemical and Physical Changes:
All matter may undergo changes, either physical or chemical.
Physical changes to matter do not change the matter’s composition.
Chemical changes to matter do change the matter’s composition.
Examples:
The Big 3: Indicators of a Chemical Change.
The Scientific Method:
The method basically lays out an organized plan to solve a problem.
This method is employed by more than simply scientists. It can be applied to most any situation.
Step 1: State the Problem |
You cannot solve a problem until you know exactly what it is.
Problem - A pitcher needs to get a batter out. |
Step 2: Research and Background |
What will it take to solve my problem? Research prior to game for all batters on other team, watching team play live or on video.
What do I need to know, about my problem? I need to know what pitches each batter has trouble with.
What do I know, about my problem, examine the possibilities? Eliminate poor choices Consider likely choices |
Step 3: Form a Hypothesis |
A possible solution to my problem. (The simplest solution is often the best solution!) Batter hits the fastball well; throw curveballs, sliders and change-ups. . Batter hits the away pitches. |
Step 4: Test the Hypothesis |
Perform an experiment to see if your hypothesis works. Throw offspeed pitches on the inside of the plate. |
Step 5: Draw Conclusions from Data Reform Hypothesis |
Data are the results of an experiment. In its simplest form, there are only two possible conclusions: Conclusion 1: If your hypothesis was correct, the batter is out. PROBLEM SOLVED!
Conclusion 2: If your hypothesis was incorrect, the experiment failed. DON'T GIVE UP! DO MORE RESEARCH! - What was wrong with your original hypothesis? - Did you make a poor selection? - Was your experiment flawed? - Form another hypothesis based on additional research. - Test the new hypothesis. |
parameter |
SI |
English |
Length |
meter |
foot |
Volume |
liters |
gallons |
Mass |
grams |
slugs/stones |
Energy |
Joule or Cal |
BTU |
Time |
sec |
sec |
Pressure |
Pascal |
psi |
Force |
Newton |
Pound |
List other units of length: m, cm, mm, km, Mm, mm, pm, ft, in, mile
Which is easier to relate? SI uses factors of 10
G |
giga |
109 |
billion |
1000000000 |
|
M |
mega |
106 |
million |
1000000 |
|
k |
kilo |
103 |
thousand |
1000 |
|
c |
centi |
10-2 |
hundredth |
.01 |
|
m |
mill |
10-3 |
thousandth |
.001 |
|
m |
micro |
10-6 |
millionth |
.000001 |
|
n |
nano |
10-9 |
billionth |
.000000001 |
|
Base Unit vs. Derived Unit:
There are two classes of Units: Base and Derived
1 milliliter = 1 centimeter cubed
1mL = 1cc = 1cm3
Exponents:
k means 1000 or 103 so 1 km means 1000 meter.
m means 1/1000 or 10-3 so 1 mm means one thousandth of a meter.
Exponents mean how many times you multiple that number to itself.
If there is a negative sign, it is the same thing but it is in the denominator of a fraction.
Example:
23 = 2 × 2 × 2
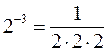
Significant Figures:
Rule 1: non-zero numbers are always significant.
Rule 2: Any zeros between two numbers are significant.
Rule 3: Trailing zeros have two rules:
A – if the trailing zero in the value is to the right of the decimal, the zero is significant.
B – if the trailing zero in the value is to the left of the decimal, the zero is only significant if it is followed by a decimal point.
How many significant figures are in the following values?
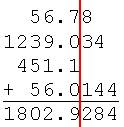
When you add or subtract numbers, the number whos significant figure falls farthest to the left controls the significant figures for your answer.
56.78
1239.034
451.1
+ 56.0144
1802.9284
You may only report 1802.9
For multipication or division answers, you may only report the number of significant figures equal to the fewest number of significant figures from any one of the values in the calculation.

So, your answer may be reported as 5400 or 5.4 x 101
Either is correct, but the scientific notation answer would be considered formated more correctly.
Unit Conversion Methods: Railroad or Dimensional Analysis or Factor Label:
This is the most effective means of handling mathematical calculations when the variables have units converting units.
Example 1: miles ® kilometers
9.8 mi |
|
|
|
|
= |
|
1 mi |
|
|
100 cm |
|
![]()
![]()
![]()
9.8 x 5280 x 12 x 2.54 x 1 x 1 |
= 15.77 km |
1 x 1 x 1 x 100 x 1000 |
|
Example 2: miles per hour ® feet per second
![]()
![]()
22 mi |
5280 ft |
1 hr |
= |
hr |
|
|
60 sec |
22 x 5280 x 1 x 1 |
= |
32.3 ft |
1 x 60 x 60 |
|
sec |
Characteristics of Phases
|
Density |
Shape |
Thermal Expansion |
Compressible |
Solid |
High |
fixed |
Very Little |
Very Little |
Liquid |
Medium |
takes shape of the container |
Very Little |
Very Little |
Gas |
VERY Low |
takes shape of the container |
Lots |
Very Easily |
Density:
Example1:
What is the density of a piece of wood with a mass of 2200 grams and a volume of 2400 cm3?
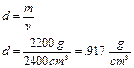
Will this piece of wood float in water? Yes, its density is less than 1g/cm3
Example 2: Calculating mass given density and volume
The equation to calculate density is D=m/v, but if you know the units of each variable you don’t need to know the equation.
The reasoning: you know the units for mass are grams or kilograms, so your answer must be in units of grams.
Set up the railroad to eliminate all units except the mass unit and make sure the mass unit is in the numerator.
D = 7.2 g/cm3
v = 35 cm3
7.2 g |
= |
|
|
7.2 x 35 |
= |
252 g |
|
|
|
Specific Gravity:
This for of measurement is another way to, for all practical purposes, another way to measure density. This method is a relativistic method, meaning you relate the density of your sample to the density of water.
This method is used a lot in the medical, wine and brewing fields. In the medical field, blood and urine samples are frequently measured in terms of their specific gravity. The alcoholic beverage industries uses specific gravity to determine the amount of alcohol in the beer or wine.
Specific gravity is easy to calculate. You divide the density of your sample by the density of water. Well the density of water is 1 g/cm3 so the calculation is kind of silly. You simple remove the units.
For example:
If density of normal urine is 1.03 g/cm3 and the density of water is 1 g/cm3
![]()
So, what did we do here? We removed the unit.
Energy:
We will talk about two definitions for energy, potential and kinetic.
What is KE? A definition is the energy of motion of a substance. This is energy being used right now. The equation would be:
![]()
Where m is the substance’s mass and v is the substance’s velocity.
So, KE depends on two things, how big the object is and how fast it is going. This is common sense. A dump truck traveling at 50mph has more energy than a VW bug going 50mph and a BMW moving at 110 mph has a lot more energy than a BMW moving at 55 mph, see the story in USAToday’s sports section dated 1/15/03 about a Dallas Cowboy’s cornerback.
So, if KE is the energy of use, potential energy, PE, is stored energy. This energy has the potential to do something, but right now it is being stored.
Look back at that dump truck. Let’s say it is parked on a hill. It has more potential energy sitting up there than if it was parked at the bottom of the hill. On the hill it has the potential to turn that PE into KE and come racing down the hill through someone’s home.
PE is not only found in position, chemicals have stored energy. The gasoline in the tank of the BMW that is driving 110 mph is not involved in making the car move; it has the potential to, and when it is pumped from the tank to the engine, its potential energy will be converted to kinetic energy. The octane in the gasoline will be converted into carbon dioxide, carbon monoxide, water and energy.
You will see the following units used to describe energy, calories and joules. A calorie is the English unit, with joules being the SI unit. The abbreviations are cal and J. A calorie is defined as that amount of energy needed to raise the temperature of one gram of water one degree Celsius. So, this is not much energy. A calorie is equal to 4.184 J, so a joule is even less energy.
There is a law that applies to this topic, the Law of the Conservation of Energy. This law states that energy is neither created nor destroyed; it only changes forms. So, PE is converted to KE and vice versa, but the energy is never really lost. This law took some heat from Einstein’s theory of relativity which is surmised by the equation E = mc2. E is energy, m is mass and c is the speed of light. Well from this equation energy can be lost, it could be converted into mass. So, is the law untrue? No, Einstein said that the law still applies as written because the scientific community holds that matter is just a form of energy, so as written we have only changed its form since it can be converted back from matter to energy.
Temperature:
Temperature is defined as a measure of the average kinetic energy of a substance. The symbol for average kinetic energy is ![]() .
.
So, if we make an object move faster its temperature goes up. This is also kind of common sense. The water molecules in ice are moving slower than the water molecules in liquid water or much slower than in steam.
Another example is a cold basketball. If you have ever pulled your ball out of the trunk of your car in December and went to bounce it, you know it does not bounce back up to you. But if you keep trying, after many bounces, the ball behaves normally. Why? You slammed the air molecules in the ball around so now they are moving faster, their temperature has actually gone up.
Lord Kelvin is honored with having the absolute temperature scale named after him. What this scale really deals with is the speed of molecules. Basically, the Zero of Absolute Zero is the point at which molecules stop moving. The reason you measure temperature is to find out what speed the molecules of a substance are moving.
English |
Fahrenheit |
Metric |
Celsius |
Metric |
Kelvin |
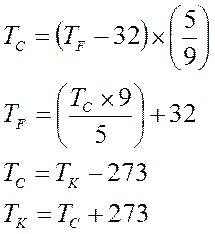
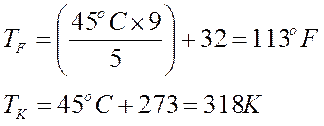
Notice Kelvin does not get the little degree circle!
These three thermometers are at the same level, meaning the average KE (temperature) of each is the same. But each thermometer is scaled differently. This is the temperature at which water freezes but each is reading a different number.
F = 32°
C = 0°
K = 273
Specific Heat:
Heat is simply a form of energy. Specific heat is a unit used to describe how much heat is needed to change the temperature of an object.
We use this principle every time you cook. The pan is made of metal, but the handle is coated in plastic, why? Metal’s temperature changes with small amounts of heat, so it transfers heat well while plastic’s temperature does not change unless a large amount of heat is added to it, it does not transfer heat well.
Another example would be the paper sleeves you get with your coffee. The cup is really hot, but if you put on the sleeve you are not burned by the cup because the sleeve is made of a material that does not transfer heat.
Specific heat is defined as that amount of heat needed to changed the temperature of one gram of a substance one degree Celsius. That sounds like the definition of a calorie, a calorie is defined as that amount of energy needed to raise the temperature of one gram of water one degree Celsius.
Here is the equation to calculate a substance specific heat capacity.

Here are some examples of substances specific heat capacities:
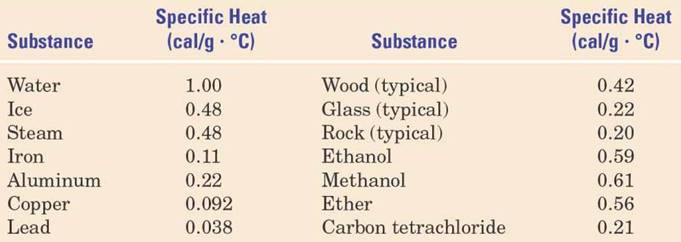
Example:
The specific heat of stone is .19 cal/g×°C. For a 600 gram sample of stone with an initial temperature of 20°C and a final temperature of 90°C, how much heat was added to the stone?
Q = |
|
.19 cal |
|
= 7980 cal |
|
|
|
|
|
An interesting point involves the calorie unit. You no doubt have heard the word calorie in terms of food. The funny little fact is that the food calorie and the science calorie are different. The science calorie is abbreviated cal the food calorie is Cal. The food calorie’s value is actually 1000 cal. So, these colas, which claim they are one calorie, are actually 1 Calorie, a thousand times more than the calorie. Read a food label, the word Calorie is capitalized.
Fonte: https://www.cgcc.edu/sites/cgcc.us/files/science-dept/chemsitry/notes/ch104/chemistry-matter-units-densit-temperature.doc
Sito web da visitare: https://www.cgcc.edu/
Autore del testo: non indicato nel documento di origine
Il testo è di proprietà dei rispettivi autori che ringraziamo per l'opportunità che ci danno di far conoscere gratuitamente i loro testi per finalità illustrative e didattiche. Se siete gli autori del testo e siete interessati a richiedere la rimozione del testo o l'inserimento di altre informazioni inviateci un e-mail dopo le opportune verifiche soddisferemo la vostra richiesta nel più breve tempo possibile.
I riassunti , gli appunti i testi contenuti nel nostro sito sono messi a disposizione gratuitamente con finalità illustrative didattiche, scientifiche, a carattere sociale, civile e culturale a tutti i possibili interessati secondo il concetto del fair use e con l' obiettivo del rispetto della direttiva europea 2001/29/CE e dell' art. 70 della legge 633/1941 sul diritto d'autore
Le informazioni di medicina e salute contenute nel sito sono di natura generale ed a scopo puramente divulgativo e per questo motivo non possono sostituire in alcun caso il consiglio di un medico (ovvero un soggetto abilitato legalmente alla professione).
"Ciò che sappiamo è una goccia, ciò che ignoriamo un oceano!" Isaac Newton. Essendo impossibile tenere a mente l'enorme quantità di informazioni, l'importante è sapere dove ritrovare l'informazione quando questa serve. U. Eco
www.riassuntini.com dove ritrovare l'informazione quando questa serve