In this section you will be required to use the following prefixes and units:
k = kilo = x 103 (3 kJ = 3 x 103 J = 3 000 J )
M = mega = x 106 (8 MW = 8 x 106 W = 8 000 000 W )
G = giga = x 109 (4 GW = 4 x 109 W = 4 000 000 000 W )
T = tera = x 1012 (2 TJ = 2 x 1012 J = 2 000 000 000 000 J )
There are a wide variety of energy units used in industry. Some examples of these are shown in the following table.
Unit |
Unit symbol |
Equivalent energy in joules |
therm |
B.T.U |
1·06 x 106 |
kilowatt hour |
kWh |
3·60 x 106 |
tonne of oil equivalent |
t.o.e |
4·76 x 1010 |
tonne of coal equivalent |
t.c.e |
2·80 x 10 1`0. |
Calorie |
kcal |
4·18 x 10 3 |
1. (a) How many joules of energy are equivalent to 1 t.c.e.?
(b) Express this answer in kJ, MJ and GJ.
2. How many joules of energy are equivalent to the following amounts of energy:
(a) 2 kWh (b) 8 B.T.U. (c) 5 kcal.
(d) 40 kWh (e) 3 x 1012 B.T.U. (f) 150 kcal.
3. A certain mint sweet contains only 2 Calories of energy. How many joules of energy are contained in this mint sweet?
4. The electricity output of a power station is 2·5 x 1016 J per year. How many therms are supplied by this power station in one year?
5. An oil-fired power station uses 1 200 tonnes of oil per day. Only 30% of the chemical energy in the oil is actually converted into electrical energy.
(a) How much energy in joules is contained in 1 200 tonnes of oil?
(b) How many joules of electrical energy are produced each day?
6. In 1993 an average citizen of the United Arab Emirates used 16·88 t.o.e. of energy while a person living in Chad used only 0·016 t.o.e. Approximately how many people from Chad together consumed the same amount of energy as 1 person from the United Arab Emirates?
7. In the United Kingdom in 1993, 2·153 x 108 t.o.e. of energy was consumed by a population of 58 million. 26 % of this energy was produced by the 35 nuclear reactors in the UK at that time.
(a) What was the average energy consumption in t.o.e. of a person living in the UK
in 1993?
(b) How much energy in t.o.e. was produced by nuclear reactors in the UK in 1993?
(c) In this year what was the average energy produced in t.o.e. by a single nuclear
reactor in the United Kingdom?
(d) How much nuclear energy was consumed, per person, in the UK in 1993? Give your answer in t.o.e.
Remember that you can use the equation:
where P = power in watts(W) From this equation we can see that: 1 watt = 1 joule per second |
8. Solar power is an attractive renewable source of energy in sunny areas of the world. One successful solar power project is based in the Mojave desert in California. Here, a very large area of desert has been transformed into solar fields. The Sun’s energy is captured by mirrored troughs. Water flows along the troughs, in pipes, and the heat from the Sun converts it to steam. One square metre of mirrored troughs can produce 150 J of electricity each second. Each solar field can power a 30 MW turbine.
(a) What area of desert is required to drive a 30 MW turbine?
(b) How many fields would be required to produce electricity at a rate of 210 MW?
(c) If 5 fields operated at full power for 1 hour how many kWh of electrical energy would they produce?
9. A typical wind turbine can produce electricity at a rate of 400 kW, while a typical thermal power station produces 1 200 MW.
(a) How many wind turbines would be required to produce electricity at the same rate as one thermal power station?
(b) In one particular year a wind turbine operated at 400 kW for a total of 90 days. In the same year a 1 200 MW thermal power station operated for a total of 270 days.
(i) How many joules of electrical energy did the wind turbine produce in this year?
(ii) How many joules of electrical energy did the thermal power station produce in this year?
(iii) How many wind turbines would have been required to produce the same amount of energy, in this year, as one thermal power station?
10. In mountainous regions of the world electricity is often produced by hydroelectric schemes. Britain’s largest pumped storage scheme, Dinorwig, operates inside a mountain in Snowdonia, Wales. This power station is capable of producing 31·14 TJ of electricity in a period of 5 hours as water crashes down from an upper lake to a lower lake.
(a) What is the maximum power which can be delivered by Dinorwig Power Station?
(b) The electricity board charges 6 pence per kWh of electricity. How much money would the electricity board make from Dinorwig in 5 hours if the power station was operating at full power?
In this section you can use the following equations:

where E = energy in joules(J)
I = current in amps (A)
t = time in seconds(s)
Ep = gravitational potential energy in joules(J)
m = mass in kilograms(kg)
g = gravitational field strength in newtons per kilogram(N /kg)
h = height in metres(m)
P = power in watts(W)
V = voltage in volts (V)
Often in these questions you will be given the rate of flow of water i.e. how many kilograms flow each second. This gives you two pieces of information, mass and time.
1. How much potential energy could be converted into electrical energy when 200 kg of water falls 40 m into a turbine attached to a generator?
2. How much energy is stored in a pumped storage hydroelectric scheme if 6 000 kg of water is pumped up 210 m to a reservoir at night time when there is a surplus of electricity?
3. In a hydroelectric power station 400 kg of water flow each second through the turbines of the power station from a loch 200 m above the turbines.
(a) How much potential energy is lost by the water each second?
(b) How much electrical energy could be generated each second assuming that there are no energy losses?
(c) What would the output power of this station be?
4. A small reservoir is situated 120 m above a hydroelectric power station. The station gives an output power of 2·25 MW.
(a) How much electrical energy is generated each second in this power station?
(b) How much potential energy must the water flowing into the turbines lose each second in order to generate this electricity?
(c) What mass of water must flow each second through the turbines of the power station in order to generate 2·25 MW of electricity?
5. The generator of a hydroelectric power station produces an output power of 1·1 MW. Water flows at a rate of 300 kg per second from the loch above the power station.
(a) How much electrical energy is generated each second?
(b) How much potential energy must be converted into electrical energy each second?
(c) How high is this loch?
6. (a) How much energy is stored in the reservoir of this hydroelectric scheme if it contains 200 000 kg of water?
(b) If all this water flows into the turbines attached to the generators in
1 hour what would the output power from this station be?
7. 2·4 x 106 kg of water flow from a loch 150 m high in 8 hours. What power could this water generate in a hydroelectric power station?
8. An electric pump is required to fill a reservoir with 1·6 x106 kg of water in 6 hours. The reservoir is 40 m above the pump house.
(a) How much potential energy must the water gain in 6 hours?
(b) How much electrical energy must the pump receive in the 6 hours?
(c) Calculate the power of the pump.
9. In a model pumped storage hydroelectric power station an electric pump is used to lift water through a height of 1 m. The pump is connected to a 12 V supply and draws a current of 2 A.
(a) How much electrical energy does the pump use each second?
(b) How much potential energy could this pump give to the water each second?
(c) What mass of water can the pump lift each second?
10. A 230 V pump drawing a current of 3 A is required to lift 200 kg of water through a height of 7 m .
(a) How much potential energy will the water gain?
(b) How much electrical energy must be provided to the pump?
(c) How long will it take for the pump to lift all the water?
In this section you can use the following two equations:

Example 1
A generator in a thermal power station converts 1 000 J of kinetic energy into 800 J of electrical energy. What is the efficiency of the generator?
efficiency = useful energy out = 800 = 0·8 = 80%
useful energy in 1000
Example 2
A motor is 65 % efficient. What power can this motor deliver when it receives
2 000 W?
65 %= 0·65 = useful power out
2 000
useful power out = 0·65 x 2 000 = 1 300 W
1. Find the missing values in the following table.
|
Efficiency (%) |
Useful energy in(J) |
Useful energy out(J) |
(a) |
|
1 400 |
700 |
(b) |
|
675 |
135 |
(c) |
80 |
1 200 |
|
(d) |
45 |
|
1 500 |
(e) |
60 |
300 |
|
(f) |
25 |
|
6 000 |
2. A coal fired power station has a power output of 200 MW. The power produced by the boiler is 340 MW. Calculate the efficiency of the power station.
3. A turbine converts 65 000 J of heat energy into 13 000 J of kinetic energy. What is the efficiency of the turbine?
4. A generator converts 3 156 MJ of kinetic energy into 450 MJ of electrical energy. What is the efficiency of the generator?
5. A thermal power station converts 420 MJ of chemical energy into 124 MJ of electrical energy. What is the efficiency of this power station?
6. An electrical pump used in a pumped storage hydroelectric power station is 80 % efficient. How much work can the pump do if it is supplied with 25 kJ of energy each second?
7. An oil fired power station which is 40% efficient produces an output of 300 MW. How much power must be supplied to the station to produce this output?
8.
The output from an oil-fired power station is 250 MW and it is 32 % efficient. How much power must be provided by the oil to produce this output?
|
9. The Glenlee hydroelectric power station produces 24 000 kW of electricity. How much power is provided by water falling from the reservoir if the station is 25 % efficient?
10. The boiler of a thermal power station releases 2·8 x 108 J of heat energy for each kilogram of coal burned. The generator of the power station produces 1·26 x 108 J of electrical energy for each kilogram of coal burned. What is the efficiency of this power station?
11. The tidal power station at the Rance in Brittany, France opened in 1966. Each of the 24 turbines can generate an output of up to 10 MW from the tidal currents funnelled into the river estuary. Assuming that each turbine is 45 % efficient calculate the power of the tide required to generate 10 MW at one turbine.
12. Water flowing into the turbines of a hydroelectric power station loses 4·5 x 106 J of potential energy each second. How much electrical energy could this power station produce if it is 35 % efficient?
13. A house has solar panels covering an area of 10 m2 to provide domestic hot water. The solar power received by each square metre is 180 W on a summer day and the panels are 20 % efficient. What would be the heat produced by the panels on such a day?
14. The average power in waves washing the north Atlantic coast of Europe is 50 kW per metre of wave front. What length of wave front would be required to generate 10 MW of electricity from these waves using a 45 % efficient wave - power device?
15. The 3 MW wind turbine at Burger Hill in Orkney provides energy for the national grid. If this turbine is 25 % efficient calculate how much energy is wasted each second in this system.
In this section you can use the equation:
![]()
where Np = number of turns in primary coil
Ns = number of turns in secondary coil
Vp = voltage in primary coil in volts(V)
Vs = voltage in secondary coil in volts(V).
1. Find the missing values in the following table.
|
Np |
Ns |
Vp (V) |
Vs (V) |
(a) |
10 |
1 |
20 |
|
(b) |
200 |
100 |
480 |
|
(c) |
50 |
150 |
|
300 |
(d) |
15 |
90 |
|
480 |
(e) |
10 |
|
60 |
240 |
(f) |
|
1 000 |
30 |
3 000 |
2. Calculate the voltage induced in the secondary coil in each of the following transformers:
(a) (b) (c)
Np=100
Np=250
Np=500
Ns=50
Ns=1 000
Ns=4 000
3. Calculate the number of turns in each of the secondary coils below:
(a) (b) (c)
Np=100
Ns=?
Np=200
Ns=?
Np=500
Ns=?
4. Calculate the primary voltage in each of the following transformers:
(a) (b) (c)
Np=120
Ns=360
Np=120
Ns=10
Np=30
Ns=3
5. Calculate the number of turns in the primary coil of each of the following transformers:
(a) (b) (c)
Np=?
Np=?
Np=?
Ns=100
Ns=40
Ns=40
6. A transformer is designed with 20 turns of wire in the primary coil and 2 000 turns in the secondary coil. If the induced voltage in the secondary coil is 4 000 V calculate the primary voltage.
7. A step down transformer changes the voltage of a signal from 360 V down to 18 V. If the transformer had 200 turns in the primary coil calculate the number of turns in the secondary coil.
8. An industrial power plant uses step up transformers to step up the voltage of signals by a factor of 30. Suggest a possible turns ratio in the primary and secondary coils to achieve this.
9. A young boys train set is designed to operate safely
|
|
10. Louise’s aunt in America sends her a ‘mega’ frying pan as a wedding gift. It is designed to operate at 115 V. Suggest a possible turns ratio for the transformer if the pan is to be operated safely at British mains voltage.
Helpful Hint Electrical power supplied to the primary coil = Ip Vp In an ideal transformer, i.e. one which we assume is 100% efficient, then we can write |
11. Find the missing values in the following table:
|
Vp (V) |
Vs (V) |
Ip (A) |
Is (A) |
(a) |
240 |
10 |
10 |
|
(b) |
240 |
120 |
5 |
|
(c) |
36 |
720 |
|
1 |
(d) |
6 |
|
60 |
10 |
(e) |
|
4 000 |
800 |
2 |
(f) |
36 |
|
1·5 |
6 |
12. Calculate the missing variable in each of the following transformers.
(a) (b) (c)
![]()
Ip=?
Is=0·18 A
Ip=2 A
Is=0·02 A
13. A transformer is used to step the voltage from 2·4 kV down to 0·6 kV. Calculate the current in the secondary coil if the primary coil carries a current of 20 A.
14. The current in the primary and secondary coils of a transformer are 0·2 A and
0·05 mA respectively. If the voltage induced in the secondary coil is 80 V calculate the size of the primary voltage.
15. Calculate the size of the induced voltage in a step up transformer given that the primary voltage is 15 V and the current decreases from 0·810 A to 0·022 5 A .
Helpful Hint
We now have two formulae linking the voltage between the primary and secondary coils of an ideal transformer.
![]() and
and ![]()
By combining these formulae we can obtain a third formula which can be useful in questions where only the number of turns and current in a transformer are given. i.e.
![]()
16. Use the above formula linking number of turns and current in a transformer to find the missing variable in the following examples:
(a) (b) (c)
Ip=20 A
Is=4 A
Ip=0·2 mA
Is=2 A

17.
A transformer is used to deliver a low voltage to a radio cassette. |
18. A transformer is used to safely operate a door bell at 6 V. If the current in the transformer increases from 50 mA to 2 A. Suggest a suitable turns ratio for the transformer which would be necessary to achieve this.
19. A car battery uses a transformer to step a voltage of 240 V down to 12 V.
If the primary current is 0·5 A and there are 200 turns in the primary coil calculate:
(a) the number of turns in the secondary coil
(b) the current through the secondary coil.
20. A step up transformer is used to operate heavy machinery in an industrial plant.
If the number of turns increases from 300 to 3 600 how do the values of:
(a) the primary and secondary voltages compare
(b) the primary and secondary currents compare.
The national grid is a network of lines and transformers which transfer electricity
around the country. The grid uses both step up transformers and step down transformers.
All the transformer formulae used in the previous sections will be useful in solving the following problems.
Power loss in transmission lines =(current )2 x resistance of lines |
Also in this section you can use the equation:
also written as
P = I2R
where P = power loss in watts(W)
I = current in amps(A)
R = resistance in ohms(W).
1. A model transmission line is shown below.
transmission
lines
transformer B
transformer A
Transformer A has 15 turns in its primary coil and 225 in its secondary coil. Calculate:
(a) the input power of transformer A given that the input current is 50 A and it operates with a voltage of 25 000 V
(b) the output power of transformer A, assuming it is 100% efficient
(c) the current through the transmission lines
(d) the voltage across the transmission lines
(e) the resistance of the transmission lines
(f) the power loss in the lines.
2. A student sets up a demonstration to show how electricity is transmitted from a power station to our homes. He has a number of different transformers to choose from.
|
Primary turns |
Secondary turns |
A |
100 |
10 |
B |
10 |
100 |
C |
100 |
100 |
(a) Which transformer is most suitable to use between the power station and transmission lines? Explain your answer.
(b) Which transformer is most suitable to use between the transmission
lines and our home? Explain your answer.
3. A section of a model transmission line is shown below.
A student investigating power loss tries out the model using different step up transformers and types of transmission lines and notes the current through each of them.

![]()
step up step down
transformer transformer
Use the results from the table below to calculate the power loss in each line.
|
current(A) |
length of lines(m) |
resistance of lines per metre(W/m) |
(A) |
10 |
20 |
0·003 |
(B) |
9·8 |
20 |
0·002 |
(C) |
10 |
20 |
0·001 |
Helpful Hint Transformers are not always 100% efficient due to heating effects in the coils etc. Efficiency can be calculated using the formula: Efficiency of transformer = useful power out |
4. Calculate the efficiency of a transformer given that the power of the primary coils is 48 W and the power of the secondary coils is 36 W.
0·5 A
10 A
 
230 V 9 V Calculate the efficiency of the transformer.
|
|
6. A transformer is used in the battery charger of a hand blender.
If the input is 230 V, 0·8 A and the induced output is 12 V, 12 A , how efficient is the transformer?
Questions 7, 8, 9 and 10 can be solved using information from a section of the national
grid shown below.(Assume all transformers are 100 % efficient)
7. The voltage from the power station has to be stepped up before transmission at
275 000 V. Calculate the number of turns in the primary coil of the transformer given
that there are 33 000 turns in the secondary coil.
8. Assuming the transformer linking the power station and transmission lines is 100% efficient calculate the power loss in the cables given that the total resistance of them is 500 W.
9. Suggest a possible set of values for the number of turns in the primary and secondary coils of the transformer linking the grid supply point and the intermediate substations.
10. Small users such as offices, shops and houses receive only 230 V. The number of turns in the primary coil of step down transformer linking the intermediate power station and the small users is 110 000. Calculate the number of turns in the secondary coil.
In this section you can use the equation:
heat energy = specific heat capacity x mass x temperature change
also written as
Eh = cmDT
Where Eh = heat energy in joules (J)
c = specific heat capacity in joules per kilogram per degree Celsius (J/kgoC)
m = mass in kilograms (kg)
DT = change in temperature (oC).
1. Find the missing values in the following table.
|
Heat energy (J) |
Specific heat capacity (J/kgoC) |
Mass (kg) |
Temperature change (oC) |
(a) |
|
4 200 |
2 |
65 |
(b) |
|
902 |
5·5 |
15 |
(c) |
2·4 x 10 3 |
386 |
1·6 |
|
(d) |
4 250 |
|
17 |
0·5 |
(e) |
1·6 x 103 |
|
1·5 |
2 |
(f) |
|
128 |
50 x 10-3 |
30 |
2. How much heat is required to raise the temperature of 3 kg of aluminium by 10 oC?
3. 3 kJ of heat is supplied to a 4 kg block of lead. What would be the rise in temperature of the block?
4. In an experiment on specific heat capacity an electric heater supplied 14 475 J of heat energy to a block of copper and raised its temperature by 15 oC. What mass of copper was used in the experiment?
5. 6·9 kJ of heat is supplied to 500 g of methylated spirit in a plastic beaker and raises its temperature by 1.5 oC. What is the specific heat capacity of methylated spirit?
6. How much heat energy would be required to raise the temperature of 2 kg of alcohol from 20 oC to 65 oC?
7. A 250 g block of copper is allowed to cool down from 80 oC to 42 oC. How much heat energy will it give out?
8. 254×4 kJ of energy are required to heat 2 kg of glycerol from 12 oC to 65 oC. What is the specific heat capacity of glycerol?
9. Which of the following would give out more heat:
A - a 2 kg block of aluminium as it cools from 54 oC to 20 oC
or
B - a 4 kg block of copper as it cools from 83 oC to 40 oC?
10. 2·5 kJ of heat is supplied to a quantity of alcohol and raises its temperature from
22 oC to 45 oC. What mass of alcohol was being heated?
11. Each concrete block in a storage heater has a mass of 1·4 kg. The blocks are heated to 85 oC at night when the electricity is cheaper and cool down during the day to 20 oC. If each block releases 77 kJ of energy during the day calculate the specific heat capacity of the concrete.
12. An immersion heater is used to heat 30 kg of water at 12 oC. The immersion heater supplies 8·6 M J of heat. Ignoring heat losses to the surroundings calculate the final temperature of the water.
13. A kettle supplies 262 k J of energy to 800 g of water in order to heat it to 90 oC. What was the temperature of the water before the kettle was switched on? |
|
14. |
A cup containing 140 g of water is heated in a microwave oven. The microwave supplies 4·9 x 104 J of heat to the water which was originally at 10 oC.
|
15. A 400 g block of lead is heated to 45 oC by an electric heater which supplies 1·2 kJ of heat. What was the initial temperature of the lead block?
In this section you can use the equation:
heat energy = mass x specific latent heat
also written as
Eh = mL
where Eh = heat energy in joules (J)
m = mass in kilograms (kg)
Helpful Hint The value of ‘L’ described above can be found in the data sheet on page 31. When you are solving a problem using this formula it is important to use the correct value of ‘L’ from the data sheet. To do this: Read the question carefully. If the question is about the change of state: liquid to gas or gas to liquid |
L = specific latent heat in joules per kilogram (J/kg).
1. Find the missing values in the following table.
|
Energy (J) |
Mass (kg) |
Specific latent heat (J/kg) |
(a) |
|
2·0 |
0·99 x 105 |
(b) |
|
35·5 |
8·3 x 105 |
(c) |
1·08 x 106 |
6·0 |
|
(d) |
4·032 x 105 |
|
11·2 x 105 |
(e) |
22·6 x 105 |
|
22·6 x 105 |
(f) |
1·837 x 108 |
550 |
|
2. Calculate the heat energy released when 2 kg of ice melts into 2 kg of water without a change in temperature.
3. How much heat energy is released when 56 kg liquid carbon dioxide changes into solid form without a change in temperature?
4. What mass of steam is produced when 7 232 000 J is supplied to water at 100 oC?
5. What mass of turpentine condenses when 168 200 J of heat energy is removed from a supply of gaseous turpentine at its boiling point?
6. Calculate the specific latent heat of fusion of aluminium given that 10×27 MJ J is required to change 26 kg of its from molten form into solid form.
7. How much heat energy is required to change 40 kg of solid carbon dioxide into liquid form with no change in temperature?
8. How much heat energy is required to evaporate 600 g of water at 100oC?
9. The melting point of a chemical substance is 137oC. How much heat is required to melt 0·7 kg of the substance at its melting point if it is known to have a specific latent heat of fusion of 1 300 J/kg?
10. How much water would evaporate if you supplied 28 500 J of energy to water at
100 o C?
11. Liquid alcohol vaporises when used to make flambees. Calculate the heat energy required to change 0·5 kg of liquid alcohol into the same mass in gaseous form when the temperature remains constant.
12. Calculate the specific latent heat of fusion of lead if it takes 500 000 J of heat to convert 20 kg of solid lead into molten form at its melting point.
13. What mass of liquid glycerol is converted to vapour if 8 300 000 J of heat energy is supplied at its boiling point?
14. A steam wallpaper stripper can be used to help the tedious manual task of preparing walls before decorating. The stripper contains 15 kg of water which turns to steam when boiled. Assuming the stripper is 100 % efficient, how much water is converted to steam after the water is boiling, if 100 x 10 5 J of energy is supplied?
15. During an experiment 0·02 kg of steam was converted to ice. The temperature was recorded at various times throughout the experiment and plotted on a graph. The graph of results is shown below.
C
A
E
F
D
B
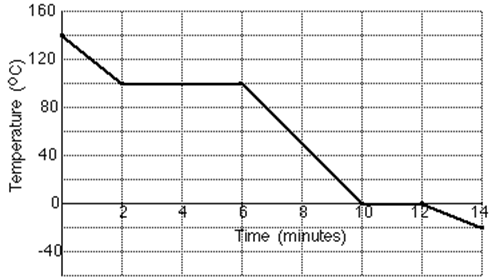
(a) Between which 2 letters on the graph is the steam changing to water?
(b) How much heat energy does the steam lose at 100 0C to become water at 100 0C?
(c) How much heat energy does the water lose at 100 0C to become water at 0 0C?
(d) How much heat energy does the water at 0oC lose to become ice?
Helpful Hint
The energy required to heat materials is often produced by an electrical heater.
Two useful equations are:
E = Pt and E = ItV
where E = energy on joules (J)
P = power in watts (W)
t = time in seconds (s)
I = current in amps (A)
You can use the principle of conservation of energy to solve problems where an electrical heater is used:
Eh = Pt = cmDT Eh = Pt = mL
Eh = ItV = cmDT Eh = ItV = mL
Use the principle of conservation of energy to solve the following:
1. How long would it take a 50 W immersion heater to heat 2 kg of water from 10 oC to 80 oC?
2. How long would it take for a 2 kW kettle to heat 800 g of water from 40 oC to 100 oC?
3. A 100 W heater is used to heat a 4 kg block of lead. If the heater is left on for
10 minutes calculate the rise in temperature of the block of lead.
4. Calculate the power of an immersion heater which takes 20 minutes to heat 4 kg of water by 60 oC.
5. An electric heater operates at 12 V and takes a current of 3 A. It is used to boil alcohol. Calculate the mass of alcohol the heater could evaporate in 20 minutes.
6. A kettle operating at mains voltage draws a current of 9×6 A. This kettle evaporated 290 g of water at 100 oC in a time of 5 minutes. What value does this give for the specific latent heat of vaporisation of water?
7. 600 g of water was supplied with 163 020 J of heat. Energy losses were negligible.
(a) What was the change in temperature of the water?
(b) If heat was supplied to the water at a rate of 543·4 joules per second, how long
did it take to heat the water?
8. The temperature of 2 kg of steel is raised by 10 0C. It takes 3 minutes for an electric heater connected to the mains (230 V) to do this.
(a) What is the minimum energy supplied to the steel?
(b) Assuming no energy losses, what is the power rating of the heater?
(c) What current is drawn by the heater?
9. A 500 g mass of copper is heated by a 40 W immersion heater while a 500 g mass of steel is heated by a 50 W heater. The initial temperature of each block is 20 0C. Which block is the first to reach a temperature of 80 oC? You can assume that there are no energy losses.
10. 80 g of alcohol at 20 0C is heated by an electric heater for 6 minutes until it reaches its boiling point of 65 oC. The heater operates at 230 V and draws a current of 125 mA.
(a) How much electrical energy is used by the heater?
(b) How much heat energy is absorbed by the alcohol?
(c) How much energy was ‘lost’ to the environment?
1. To live an active life we need energy. This energy comes from the food we eat. We also need energy to run our homes, industries and transport systems. This energy comes mostly from fossil fuels but some of it comes from renewable sources of energy. In recent years we have been asked to conserve (save) energy.
(a) Why have scientists been developing power stations that use renewable
sources of energy?
(b) Which of the following energy sources are renewable?
uranium wind coal natural gas water sunlight oil
(c) Describe two different ways to conserve energy in transport.
2. About 30 % of the energy we use in this country is in the form of electricity. Electricity is generated in power stations around the country. In Scotland we have different kinds of power stations including nuclear and hydroelectric power stations.
(a) Give one advantage and one disadvantage of nuclear power stations.
(b) What are the advantages of a pumped storage hydroelectric power station?
(c) How much potential energy could be converted into electrical energy when
200 kg of water falls 40 m into the turbine attached to the generator of a
hydroelectric power station?
3. Transformers are an important part of the National Grid System.
(a) What are transformers used for?
(b) Describe the structure of a transformer.
(c) A transformer has 2 turns of wire in the primary coil and 2 000 turns in the
secondary coil. If the induced voltage in the secondary coil is 230 000 V
calculate the primary voltage.
4. Electricity and gas bills can be very high during the winter months as people try to keep their houses warm. A lot of the heat produced from the gas and electricity is lost.
(a) In what ways is heat lost from a house?
(b) The rate at which heat is lost is much greater in winter than in summer. Explain
why.
(c) Describe two ways in which heat losses due to convection could be reduced.
(d) A concrete block of mass 1∙4 kg used in a storage heater requires 77 kJ of energy
to raise its temperature by 65 oC. What is the specific heat capacity of the
concrete?
5. When a liquid evaporates it takes in energy. If the gas is compressed it will change back to a liquid and heat energy will be given out. This idea can be used when a liquid is pumped around a closed circuit of pipes i.e. heat can be taken in and given out at different places.
(a) What name is given to the heat energy given out when a gas condenses to a
liquid?
(b) Freon is a liquid which boils at -29 oC. What happens to the temperature of Freon
as it changes to a gas?
(c) Give an example of an appliance that uses a heat pump like that described
above.
Speed of light in materials |
|
Speed of sound in materials |
|||||||||||||||
Material |
Speed in m/s |
|
Material |
Speed in m/s |
|||||||||||||
Air |
3 x 108 |
|
Aluminium |
5 200 |
|||||||||||||
Carbon dioxide |
3 x 108 |
|
Air |
340 |
|||||||||||||
Diamond |
1×2 x 108 |
|
Bone |
4 100 |
|||||||||||||
Glass |
2×0 x 108 |
|
Carbon dioxide |
270 |
|||||||||||||
Glycerol |
2.1 x 108 |
|
Glycerol |
1 900 |
|||||||||||||
Water |
2×3 x 108 |
|
Muscle |
1 600 |
|||||||||||||
|
|
|
Steel |
5 200 |
|||||||||||||
Gravitational field strengths |
|
Tissue |
1 500 |
||||||||||||||
|
Gravitational field strength on the surface in N/kg |
|
Water |
1 500 |
|||||||||||||
|
|||||||||||||||||
Earth |
10 |
|
Specific heat capacity of materials |
||||||||||||||
Jupiter |
26 |
|
Material |
Specific heat capacity in J/kgoC |
|||||||||||||
Mars |
4 |
||||||||||||||||
Mercury |
4 |
|
Alcohol |
2 350 |
|||||||||||||
Moon |
1×6 |
|
Aluminium |
902 |
|||||||||||||
Neptune |
12 |
|
Copper |
386 |
|||||||||||||
Saturn |
11 |
|
Glass |
500 |
|||||||||||||
Sun |
270 |
|
Glycerol |
2 400 |
|||||||||||||
Venus |
9 |
|
Ice |
2 100 |
|||||||||||||
Uranus |
11×7 |
|
Lead |
128 |
|||||||||||||
Pluto |
4×2 |
|
Silica |
1 033 |
|||||||||||||
|
|
|
Water |
4 180 |
|||||||||||||
|
Steel |
500 |
|||||||||||||||
Specific latent heat of fusion of materials |
|
|
|
||||||||||||||
Material |
Specific latent heat of fusion in J/kg |
|
Melting and boiling points of materials |
||||||||||||||
Material |
Melting point in oC |
Boiling point in oC |
|||||||||||||||
Alcohol |
0×99 x 105 |
|
|||||||||||||||
Aluminium |
3×95 x 105 |
|
Alcohol |
-98 |
65 |
||||||||||||
Carbon dioxide |
1×80 x 105 |
|
Aluminium |
660 |
2470 |
||||||||||||
Copper |
2×05 x 105 |
|
Copper |
1 077 |
2 567 |
||||||||||||
Glycerol |
1×81 x 105 |
|
Glycerol |
18 |
290 |
||||||||||||
Lead |
0×25 x 105 |
|
Lead |
328 |
1 737 |
||||||||||||
Water |
3×34 x 105 |
|
Turpentine |
-10 |
156 |
||||||||||||
|
|
|
|
||||||||||||||
|
|
SI Prefixes and Multiplication Factors |
|||||||||||||||
Specific latent heat of vaporisation of materials |
|
Prefix |
Symbol |
Factor |
|||||||||||||
|Material |
Sp.l.ht vap(J/kg) |
|
giga |
G |
1 000 000 000=109 |
||||||||||||
Alcohol |
11×2 x 105 |
|
mega |
M |
1 000 000 =106 |
||||||||||||
Carbon dioxide |
3×77 x 105 |
|
kilo |
k |
1 000 =103 |
||||||||||||
Glycerol |
8×30 x 105 |
|
milli |
m |
0×001 =10-3 |
||||||||||||
Turpentine |
2×90 x 105 |
|
micro |
m |
0×000 001 =10-6 |
||||||||||||
Water |
22×6 x 105 |
|
nano |
n |
0×000 000 001=10-9 |
||||||||||||
Section 1 - Supply and Demand
Prefixes and Units (p.2)
1.
(a) 2×8 x 1010 J
(b) 2×8 x 107 kJ
2.
(a) 7×2 x 106 J
(b) 8×48 x 106 J
(c) 20 900 J
(d) 1×44 x 108 J
(e) 3×18 x 1018 J
(f) 627 000 J
3. 8 360 J
4. 2×36 x 1010 therms
5.
(a) 5×71 x 1013 J
(b) 1×71 x 1013 J
6. 1 055 people
7.
(a) 3×71 t.o.e.
(b) 55 978 000t.o.e.
(c) 1 599 371 t.o.e.
(d) 0×97 t.o.e.
8.
(a) 2 x 105 m2
(b) 7 fields
(c) 150 000 kWh
9.
(a) 3000
(b) (i) 3×1 x 1012 J
(ii) 2×8 x 1016 J
(iii) 9 004
10.
(a) 1×73 x 109 W
(b) £ 519 000
Energy Transformations (p.5)
1. 80 000 J
2. 12 600 000 J
3.
(a) 800 000 J
(b) 800 000 J
(c) 800 000 W
4.
(a) 2×25 x 106 J
(b) 2×25 x 106 J
(c) 1875 kg
5.
(a) 1×10 x 106 J
(b) 1×10 x 106 J
(c) 367 m
6.
(a) 3 x 108 J
(b) 83 333 W
7. 125 000 W
8.
(a) 6×4 x 108 J
(b) 6×4 x 108 J
(c) 29 630 W
9.
(a) 24 J
(b) 24 J
(c) 2×4 kg
10.
(a) 14 000 J
(b) 14 000 J
(c) 20×3 s
Efficiency(p.8)
1.
(a) 50 %
(b) 20 %
(c) 960 J
(d) 3 333 J
(e) 180 J
(f) 24 000 J
2. 58×8 %
3. 20 %
4. 14×26 %
5. 29×5 %
6. 20 kJ
7. 750 MW
8. 781×25 MW
9. 96 000 kW
10. 45 %
11. 22×22 MW
12. 1 575 000 J
13. 360 W
14. 444×4 m
15. 9 MJ
Transformers(p.11)
1.
(a) 2 V
(b) 240 V
(c) 100 V
(d) 80 V
(e) 40
(f) 10
2.
(a) 240 V
(b) 192 V
(c) 24 V
3.
(a) 200
(b) 20
(c) 12×5
4.
(a) 120 V
(b) 144 V
(c) 480 V
5.
(a) 10
(b) 800
(c) 5
6. 40 V
7. 10
8. 1 : 30
9. 10
10. 2 : 1
11.
(a) 240 A
(b) 10 A
(c) 20 A
(d) 36 V
(e) 10 V
(f) 9V
12.
(a) 1×5 A
(b) 0×01 A
(c) 1×2 V
13. 80 A
14. 20 V
15. 540 V
16.
(a) 20
(b) 120
(c) 0×11 µA
17. 4 A
18. 40 : 1
19.
(a) 10
(b) 10 A
20.
(a) Vs = 12 Vp
(b) Ip = 12 Is
National Grid(p.15)
1.
(a) 1 250 000 W
(b) 1 250 000 W
(c) 3×33 A
(d) 375 000 V
(e) 112 612×61 W
(f) 1 248 750 W
2.
(a) B - step up
(b) A - step down
3. A : 6 W
B : 3×84 W
C : 2 W
4. 75 %
5. 78×26 %
6. 78×3 %
7. 3 000
8. 31×25 MW
9. Suitable values would be
Np= 2 913;Ns=1 000.
10. 2 200 turns
Section 4 - Heat
Specific Heat Capacity (p.19)
1.
(a) 546 000 J
(b) 74 415 J
(c) 3×89 0C
(d) 500 J/kg0C
(e) 533×3 J/kg0C
(f) 192 J
2. 27 060 J
3. 5×86 0C
4. 2×5 kg
5. 9 200 J/kg0C
6. 211 500 J
7. 3 667 J
8. 2 400 J/kg0C
9. B
10. 0×05 kg
11. 846×15 J/kg0C
12. 80×58 0C
13. 11×65 0C
14. 93×73 0C
15. 21×56 0C
Specific Latent Heat (p.21)
1.
(a) 198 000 J
(b) 29 465 000 J
(c) 180 000 J/kg
(d) 0×36 kg
(e) 1 kg
(f) 334 000 J/kg
2. 668 000 J
3. 10 080 000 J
4. 3×2 kg
5. 0×58 kg
6. 3×95 x 105 J/kg
7. 7×2 x 106 J
8. 1×36 x 106 J
9. 910 J
10. 0×01 kg
11. 560 000 J
12. 0×25 x 105 J/kg
13. 10 kg
14. 4×42 kg
15.
(a) BC
(b) 45 200 J
(c) 8 360 J
(d) 6 680 J
Conservation of Energy(p.24)
1. 11 704 s
2. 100×32 s
3. 117×19 0C
4. 836 W
5. 0×04 kg
6. 22×8 x 105 J
7.
(a) 65 0C
(b) 300 s
8.
(a) 10 000 J
(b) 55×56 W
(c) 0×24 A
9. Copper
10.
(a) 10 350 J
(b) 8 460 J
(c) 1890 J
Revision Questions
General Level(p.26)
2.
(c) 80 000 J
3.
(c) 230 V
4.
(d) 846×15 J/kg0C
Credit Level(p.28)
1.
(a) 5 000 W
(b) 1×58 x 1011 J
(c) 1 500 s
(d) 3 x 10-3 s
2.
(a) 3×65 J
(b) 0×41 J
(c) 11×2 %
3.
(b) 0×83 kg
(c) 290 0C
4.
(b) 0×2 A
5.
(a) 1 x 107 J
(b) 76 %
(c) 400 kg
6.
(a) 1×485 x 106 J
(b) 564 300 J
(c) 920 700 J
(d) 38 %
©GMV Science.
Source: http://www.kgsorkney.com/uploads/1/4/9/3/14935550/ultimatephysics_energymatters.doc
Web site to visit: http://www.kgsorkney.com
Author of the text: indicated on the source document of the above text
If you are the author of the text above and you not agree to share your knowledge for teaching, research, scholarship (for fair use as indicated in the United States copyrigh low) please send us an e-mail and we will remove your text quickly. Fair use is a limitation and exception to the exclusive right granted by copyright law to the author of a creative work. In United States copyright law, fair use is a doctrine that permits limited use of copyrighted material without acquiring permission from the rights holders. Examples of fair use include commentary, search engines, criticism, news reporting, research, teaching, library archiving and scholarship. It provides for the legal, unlicensed citation or incorporation of copyrighted material in another author's work under a four-factor balancing test. (source: http://en.wikipedia.org/wiki/Fair_use)
The information of medicine and health contained in the site are of a general nature and purpose which is purely informative and for this reason may not replace in any case, the council of a doctor or a qualified entity legally to the profession.
The following texts are the property of their respective authors and we thank them for giving us the opportunity to share for free to students, teachers and users of the Web their texts will used only for illustrative educational and scientific purposes only.
All the information in our site are given for nonprofit educational purposes
The information of medicine and health contained in the site are of a general nature and purpose which is purely informative and for this reason may not replace in any case, the council of a doctor or a qualified entity legally to the profession.
www.riassuntini.com